ACID FAST STAIN:-
• A few types of bacteria, such as the mycobacteria and Nocardia species, do not stain using common staining techniques or, if stained, they produce a variable reaction because their walls are not permeable to the rosaniline dyes in common staining regimens.
• The cell walls of the mycobacteria contain mycolic acids giving the
cell walls a high lipid content because of which these bacteria are difficult to stain.
• Visualization of these cells in samples require higher
concentrations of the dye solution and/or a heating period.
• The expression “acid fast” is derived from the observation that even with the addition of hydrochloric acid to the alcohol decolorizer, some of the stained cells retain the primary stain (carbolfuchsin)
• Cells that release the primary stain (carbolfuchsin) with decolorizing will be visible after the counterstaining step is complete.
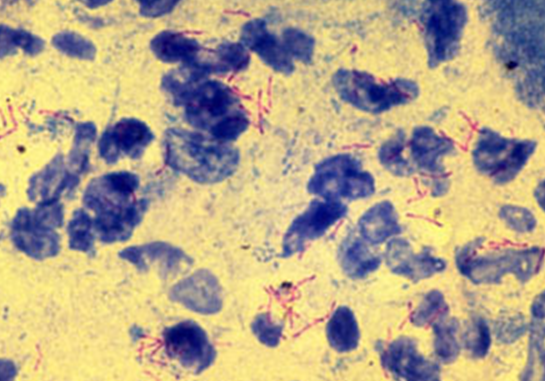
• Bacteria described as acid fast will appear red when examining
specimens using bright-field microscopy.
• Non-acid-fast cells and field debris will appear blue.
• Acid fastness is a characteristic that is shared by just a few organisms, so staining to determine if organisms possess this trait is useful in microbial identification schemes.
ACID-FAST STAINING: HIDTORICAL BACKGROUND:-
• In 1882, Robert Koch discovered the tubercle bacillus and described
the appearance by using a complex staining procedure.
• Franz Ziehl was the first to use carbolic acid (phenol) as the
mordant.
• The acid-fast stain is performed on samples to demonstrate the
characteristic of acid fastness in certain bacteria and the cysts of Cryptosporidium
and Isospora.
• Clinically, the most important application is to detect Mycobacterium
tuberculosis in sputum samples to confirm or rule out a diagnosis of
tuberculosis in patients.
• Friedrich Neelsen kept Ziehl’s mordant, but changed the primary
stain to the basic fuchsin (first used by Ehrlich in 1882). This method is
known as the Ziehl-Neelsen method.
• In this method heat is used to help drive the primary stain into the
waxy cell walls of these difficult-to-stain cells because of which the
technique is called the “hot staining” method.
• In 1915, Kinyoun published a method that has become known as the “cold staining” method because the heating step was removed in favor of using a higher concentration of the carbolfuchsin primary stain.
COMMON ACID-FAST STAINING METHOD:-
• Three methods of acid fast
staining:
– Ziehl-Neelsen (hot),
– Kinyoun (cold), and
– Auramine-Rhodamine
Fluorochrome (Truant method).
• The slides produced by
Ziehl- Neelsen and the Kinyoun methods can be visualized using a standard
bright-field microscope.
.The fluorochrome method is used by large laboratories that have a fluorescent (ultraviolet) microscope.
Ziehl-Neelsen or (Hot) method for acid-fast staining:-
• Requirements:-
– Carbolfuchsin stain:
• Basic fuchsin, 0.3 g
• Ethanol, 95% (vol/vol),
• 10 ml Phenol, heat-melted crystals, 5 ml
• Distilled water, 95 ml
Dissolve the basic fuchsin in the ethanol; then add the phenol dissolved in the water. Mix and let stand for several days. Filter before use.
– Decolorizing solvent:
• Counterstain:
– Methylene blue
Procedure:-
•
Heat fix an air dried smear at 80ͦͦC for at least 15 minutes or for 2 hours on an electric hot plate at 65ᶱC-70ᶱC.
• Place a slide with an air-dried and heat-fixed smear on
suitable staining device. Cut a piece of absorbent paper to fit the slide and saturate the paper with the carbolfuchsin stain. Or cover the smear with
carbolfuchsin.
• Keep the preparation moist with stain and steaming for 5
minutes.
• Wash the film in a gentle and indirect stream of tap water
until no color appears in the effluent.
• Repeat the decolorizing and the washing until the stained smear appears faintly pink and the fluid washing off the slide runs clear.
• Flood the smear with the methylene blue counterstain for 20 to 30 seconds, and wash with tap water.
• After air drying, examine under oil immersion. Acid-fast bacteria appear red, and non-acid-fast bacteria
appear blue.
2.Kinyoun (cold) method for acid-fast staining
• Kinyoun carbolfuchsin solution:
– Solution A: Dissolve 4 g of basic fuchsin in 20 ml of ethyl alcohol.
– Solution B: Dissolve 8 g of phenol (melted) in 100 ml of distilled water.
– Mix solutions A and B together and allow to stand for a few days.
• Acid-alcohol decolorizing agent:
– 97 ml Hydrochloric acid (concentrated), 3 ml
• Methylene blue counterstain:
– Methylene blue chloride,
0.3 g Distilled water, 100 ml Dissolve by shaking.
Procedure:-
•
Flood slides with Kinyoun’s carbolfuchsin reagent and allow
to stain for 5 minutes at room temperature.
• Rinse with deionized water and tilt slide to drain.
• Decolorize with acid-alcohol for 3 minutes and rinse again
with deionized water.
• Redecolorize with acid-alcohol for 1-2 minutes or until no
more red color runs from the smear.
• Rinse with deionized water and drain standing water from the
slide surface by tipping the slide.
• Flood slide with methylene blue counterstain and allow to
stain for 4 minutes.
• Rinse with distilled water and allow to air dry.
• Examine under high dry (400X) magnification, and confirm acid-fast structures under oil immersion (1000X)
Auramine-Rhodamine Fluorochrome or Truant method for acid-fast staining
•
Fluorescent staining reagent:
– Auramine O, CI 41000, 1.50 g
– Rhodamine B, CI 749, 0.75 g Glycerol,
– 75 ml Phenol (heat melted crystals),
– 10 ml Distilled water, 50 ml
• Mix the two dyes well with 25 ml of the water and the phenol.
Add the remaining water and glycerol and mix again. Filter the resulting
staining fluorescent reagent through glass wool and store at 4°C or room
temperature.
• Decolorizing solvent:
– Ethanol, 70% (vol/vol),
– 99.5 ml Hydrochloric acid (concentrated), 0.5 ml
• Counterstain:
– Potassium permanganate, 0.5 g
– Distilled water, 99.5 g
Procedure:-
• Flood slides with Fluorescent staining reagent and allow to
stain for 5 minutes at room temperature.
• Rinse with deionized water and tilt slide to drain.
• Decolorize with acid-alcohol for 3 minutes and rinse again
with deionized water.
• Redecolorize with acid-alcohol for 1-2 minutes or until no
more red color runs from the smear.
• Rinse with deionized water and drain standing water from the
slide surface by tipping the slide.
• Flood slide with methylene blue counterstain and allow to
stain for 4 minutes.
• Rinse with distilled water and allow to air dry.
• Examine under high dry (400X) magnification, and confirm acid-fast structures under oil immersion (1000X)