Blood bank notes
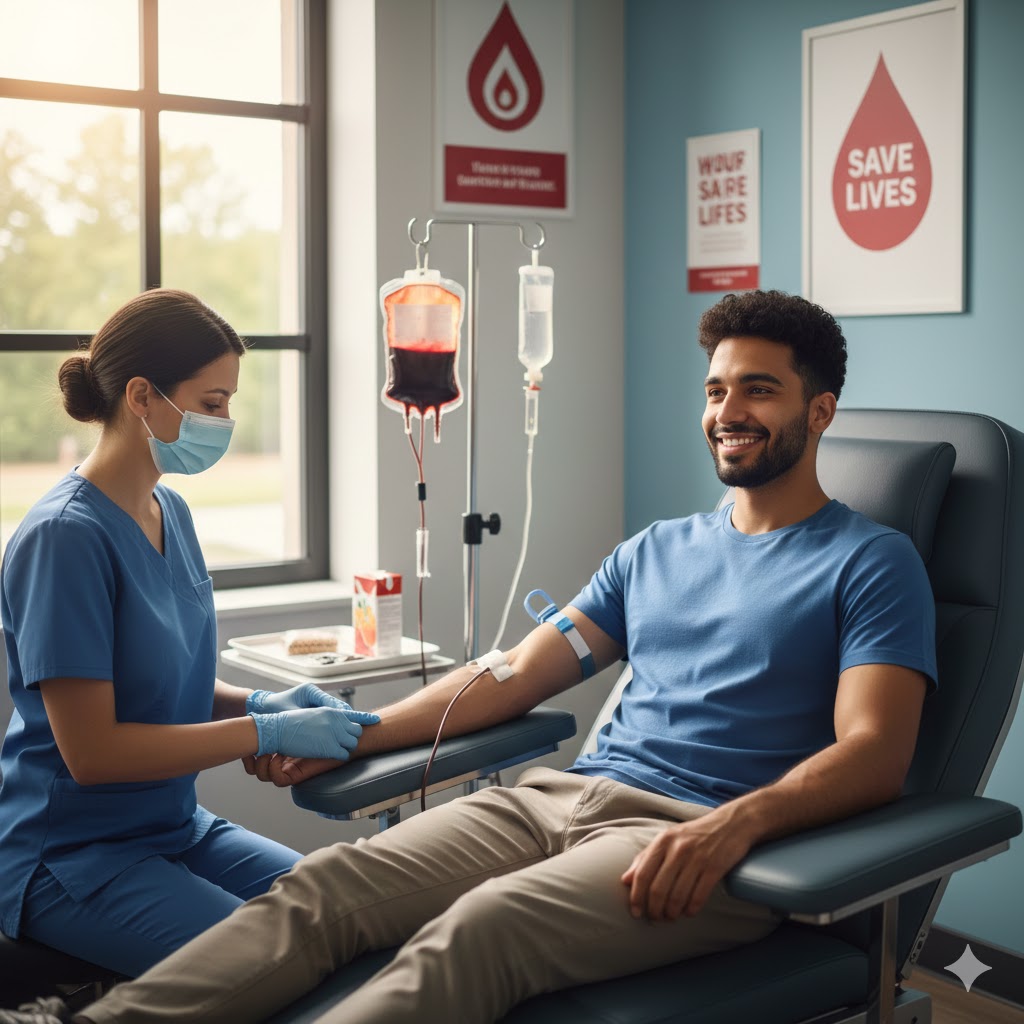
Blood banking index:- 1. Blood Bank Management & Documentation of Packaging:- Reception, Indexing & Recording Decontamination of blood bags, workbench, andal and immune antibodies) Distribution of ABO antigens (A, B, and H Antigens) on red cells and antibodies in the serum. Rh blood group system Sources of errors in ABO grouping instruments Sterilization of transfusion sets (physical & Chemical) 2. Discovery of Human Blood Group:- Theory of inheritance and nomenclature of ABO and Rh blood group system Subgroup of ABO system and Bombay group. 3. Technique for Determination of Various Blood Groups and Rh factors:- Determination of various blood groups (NaturABO hemolytic disease 4. Cross Matching and Complications of Blood Transfusion Cross math Immunological complications, non-immunological complications. History of blood bank:- The history of Blood Bank began in 1616 when William Harvey Discovered that blood circulation through the body. In 1665, a transfusion of blood from lamp saved a young patient’s life. This led to- animal to human transfusion becoming common. The first blood bank was established in a Leningrad hospital in 1932. IN 1937, Bernard Fantus was the director of the therapeutics at cook county hospital in Chicago. Established the first hospital blood bank in the United States. Fantus created a hospital laboratory that preserved refrigerated and stored donor blood. He also coined the term “blood bank”. The first blood bank in India was established in Kolkata in March 1942. The red cross managed the blood bank at all India institute of Hygiene and public health. The first successful transfusion of human blood to a patient was performed in 1818 by British obstetrician James Blundell. Blundell’s transfusion was to treat postpartum hemorrhage. In 1901, three main blood groups were discovered in 1902, the blood group is discovered in 1907. Cross matching was first used in 1914; the first non-directed transfusion was performed. In 1917, the first blood depot was established -: Universal safety rules for blood bank technicians: – Working in blood bank is a noble profession that’s required almost care and precautions. Blood bank technicians play a crucial role in ensuring the safety of both donors and recipients. To maintain a lifesaving legacy, it is essential for these technicians to follow universal safety rules. Personal protective equipment (PPE): – Always wearing appropriate PPE including Gloves Lab Coat / Gown / Apron Face Protection Head Cover / Cap Shoe Covers / Closed Shoes Additional PPE (when needed) Safety goggles When handling blood and blood products. 1.Hand hygiene: – Hand hygiene is one of the most critical infection control practices in blood bank, since staff handle human blood, component, and samples that may carry infectious agents like HIV, HCV. Wash your hands frequently and thoroughly with soap and water, apply 3-5 ml of sanitizer, rub for 20-30 second until dry should contain 60-95% alcohol. 2. Sharp object safety: – Prefer safety engineered needles and syringes with retractable or shielded tips. Do not recap needles after use. Avoid passing sharps directly from hand to hand (use trays). Dispose of used needles, scalpels, and glass pieces immediately after use in puncture-proof, leak-proof, clearly labelled sharps containers. Keep the blood collection area clean, uncluttered, and well-lit. Handle glass blood bottles, vacutainers, and pipettes carefully to prevent breakage. Regular staff training on safe handling, disposal, and emergency procedures. Posters and reminders in the blood bank to reinforce best practices. 3.Infection control:- Follow standard precaution to prevent the spread of infectious disease treat all blood product are potentially infectious. 4. Labelling: – Ensure proper labelling of all blood products and specimens confirm the information and levels match the requisition forms. 5..Blood typing and cross match: -Double check patients’identification and blood compatibility before transfusion to prevent error. Blood typing is the process of determining a person’s blood group based on the presence or absence of specific antigens on red blood cells (RBCs). Crossmatching is done before a blood transfusion to ensure compatibility between donor and recipient blood. It prevents haemolytic transfusion reactions. 6. Blood donation: – blood donation is the voluntary process of giving blood, which is then used for transfusions or to make blood components (like plasma, platelets, or red blood cells) for patients in need. If involved in the collection process, ensure that all blood donation equipment sterile and used according to established procedures 7. Equipment maintenance: – Regularly inspect and maintained equipment like refrigerator, freezer, and centrifuge to ensure the integrity of blood products 8.Storage:- Follow strict instructions of guidelines for storing blood products ensure proper temperature control and monitor for any signs of spoilage or contamination. 9.Emergency procedures: – Stay calm and follow the laboratory’s Standard Operating Procedures (SOPs) Protect yourself first using PPE (gloves, mask, apron) Alert staff and supervisor immediately Document the incident properly 10.Disposal: – Dispose of biohazardous waste including contaminants materials and sharps, accordance with rule -regulation and guidelines. 11.Training and education: – Stay current with blood banking procedures and safety practices throughout the regular training and continuing education. 12.Quality control:- Participate in quality control and assurance programs to maintain the highest standards of safety and accuracy. 13.Documantaion: – Maintain accurate records of all blood product handling testing and transfusions. Document any incidents or deviations from standards procedures 14. confidentially: – Maintain strict confidently of patient information and blood donor records. 15.Communication: – Clearly and accurately communicate with healthcare providers and other staff involved in the blood transfusion process. 16.Safety data sheets (SDS): – Familiarize yourself with safety data sheets for all chemicals and reagents used in the blood bank follow safety instructions and guidelines. 17. zero tolerance for risky behaviour: – Report any unsafe practices or deviations from safety protocols to your supervisor immediately 18.contnuous monitoring: – Regularly monitor and assess safety practices to identify and address potential risks and improvement. Donor selection:- Pre-donation counselling by trained staff should be made available maintaining privacy and confidentiality pre donation information should be included. Modes of transmission leading to risk behaviour and self-exclusion for patients’ safety. Alternative testing site Test carried out on donated blood.